Eyenovia’s Pupil-Dilating Spray Receives FDA Approval in the US
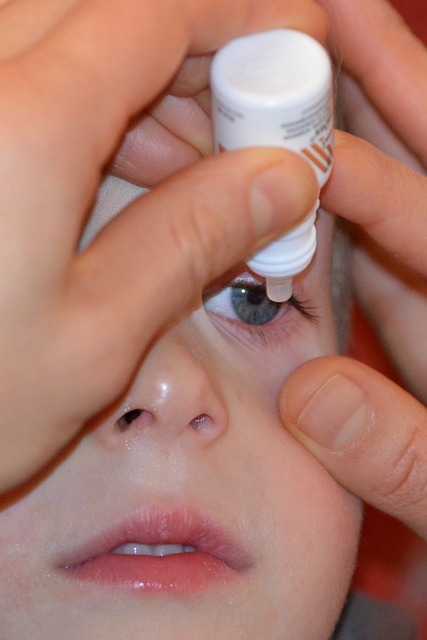
Eyenovia, pupil-dilating spray, FDA approval, ophthalmic company, eye exams, internal structures, microdosing technology, rapid onset of action, shorter duration, diagnosis, monitoring, diabetic retinopathy, age-related macular degeneration, glaucoma, patient experience, healthcare professionals, ophthalmic innovation, diagnostic toolsEyenovia, a clinical stage ophthalmic company, has received approval from the US Food and Drug Administration (FDA) for its pupil-dilating spray. The innovative product is designed to facilitate eye exams by expanding the pupil, providing a clearer view of the eye’s internal structures.
The pupil-dilating spray, known as MicroStat, is delivered via a proprietary microdosing technology that enables precise dosing and a rapid onset of action. The product’s unique formulation allows for a shorter duration of pupil dilation, reducing the time needed for eye exams and minimizing the inconvenience and discomfort associated with traditional dilation methods.
MicroStat is expected to be particularly useful in the diagnosis and monitoring of eye diseases such as diabetic retinopathy, age-related macular degeneration, and glaucoma, which require regular eye exams. By enabling clearer and more accurate images of the eye’s internal structures, the product can help doctors to detect and monitor these conditions more effectively.
The approval of MicroStat by the FDA marks a significant milestone for Eyenovia and represents a major step forward in ophthalmic care. The product’s innovative microdosing technology and fast-acting formulation are expected to improve the patient experience and increase the efficiency of eye exams, ultimately benefiting both patients and healthcare professionals.
Eyenovia is now working to bring MicroStat to market and expects to make the product available to patients in the near future. With the approval of its pupil-dilating spray, the company is well-positioned to establish itself as a leader in ophthalmic innovation and provide patients with access to new and improved diagnostic tools.